Fluicell’s R&D team is demonstrating important progress in the Company’s type 1 diabetes therapeutics in vitro development and is now able to bioprint up to 200 islet-like insulin-producing microtissues in one experimental run. The amount corresponds to the number of transplanted islets that has been demonstrated in scientific literature to have a beneficial effect in vivo in mice. The progress constitutes a crucial step towards obtaining candidates for in vivo testing, planned to commence in 2024.
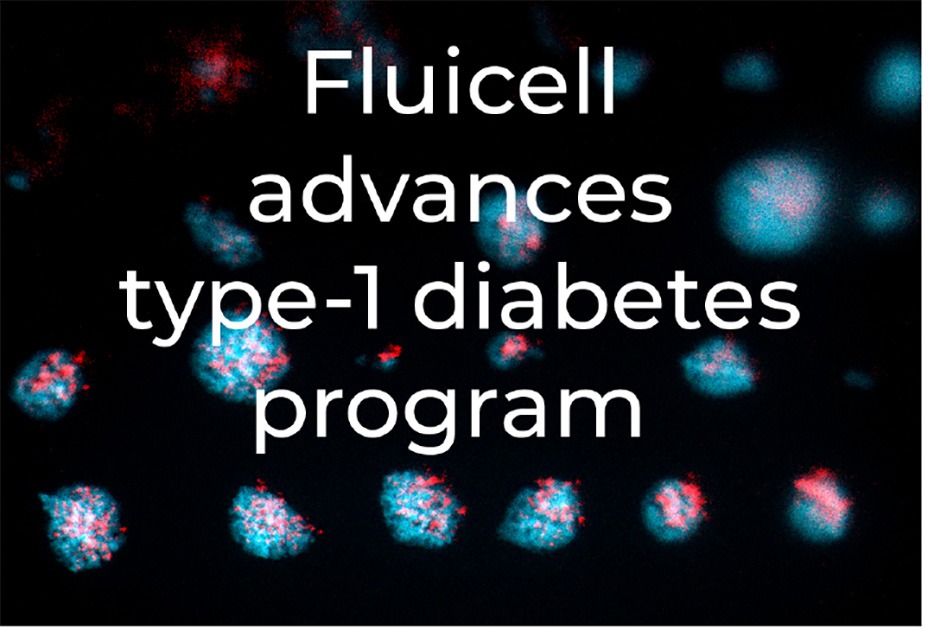
The islet-like microtissues are intended for use in the Company’s planned in vivo experiments and are of comparable size to native pancreatic islets. Moreover, the islet-like microtissues, which are bioprinted directly on transplantable material, produce insulin in response to glucose stimulation at adequate levels.
We are now creating an amount of islet-like microtissues that corresponds to what is reported in studies on diabetes reversal in mice. We are already hitting many of our development targets that we have for this year, and we are excited about our progress. Right now, we are in advanced discussions on how to initiate in vivo testing and look forward to taking the project to the next step.
Fluicell Chief Scientific Officer, Dr. Tatsiana Lobovkina
About Fluicell’s therapeutic and in vitro model development
Fluicell is conducting development of advanced therapy medicinal products (ATMP) and human tissue-based disease models for drug development. Within the disease area type 1 diabetes, Fluicell is conducting therapeutic development based on insulin-producing islet-like microtissues. Fluicell’s
development activities are based on the high precision bioprinting platform Biopixlar and its ability to generate functional tissues with high precision.
