Climate∞
In situTEM

Catalysts accelerate chemical reactions leading to higher reaction efficiencies. Observation of real time kinetics provides a clearer idea and sometimes new insights into how they actually work. Using a system like the DENSsolutions Climate allow you to observe the reaction dynamics at the micro or nano scale in real time, in a controlled environment that can be tailored to closely mimic real operating conditions. At TEM resolution, single particles can be studied which is not possible using other techniques.
DENSsolutions also offer a Gas Analyser specifically designed to work with the Climate is specifically designed to work seamlessly with the Climate in situ Gas & Heating solution. It enables analysis of reaction products, it transforms the Climate into a unique platform combining TEM-based data with information about the kinetics of the reaction under examination.
The Climate platform is also compatible with EDS detectors for chenical analyses.
The following sections illustrate a number of different imaging and analytical modes made possible by the DENSsolutions Climate in situ TEM platform that relate directly to catalyst research.
This video clearly shows the 3 stages of catalytic reaction of the nickel catalyst:
This video reveals unexpected and exciting behaviour that occurs that may not be limited to the surface of the catalyst.
Experimental data – Dr. Marc Willinger & Dr. Ramzi Farra, Fritz-Haber-Institute fur der Max-Planck-Gesellschaft, Germany.
Understanding catalyst mechanics requires observation of changes in crystal structure. In situ TEM selected area electron diffraction (SAED) enables users to study dynamic crystalline evolution on single particles.
Synchrotrons can perform similar studies using in situ XRD, but data is generated from a large number particles.
Experiment: Ni exposed to 500 mBar He:H2:O2 at 730°C, data provided by Dr. Marc Willinger & Dr. Ramzi Farra, Fritz-Haber-Institute fur der Max-Planck-Gesellschaft, Germany.
Atomic resolution imaging reveals how the surface of the material with a certain orientation shows oscillatory behaviour while other surfaces are not active which is crucial in the design of high efficiency catalysts for optimal reactive productivity.
Experiment: Cu exposed to 500 mBar H2:N2:O2 at 350 °C with data from Dr. Marc Willinger & Dr. Ramzi Farra, Fritz-Haber-Institute fur der Max-Planck-Gesellschaft, Germany & Dr. Qiang Xu, DENSsolutions, The Netherlands.
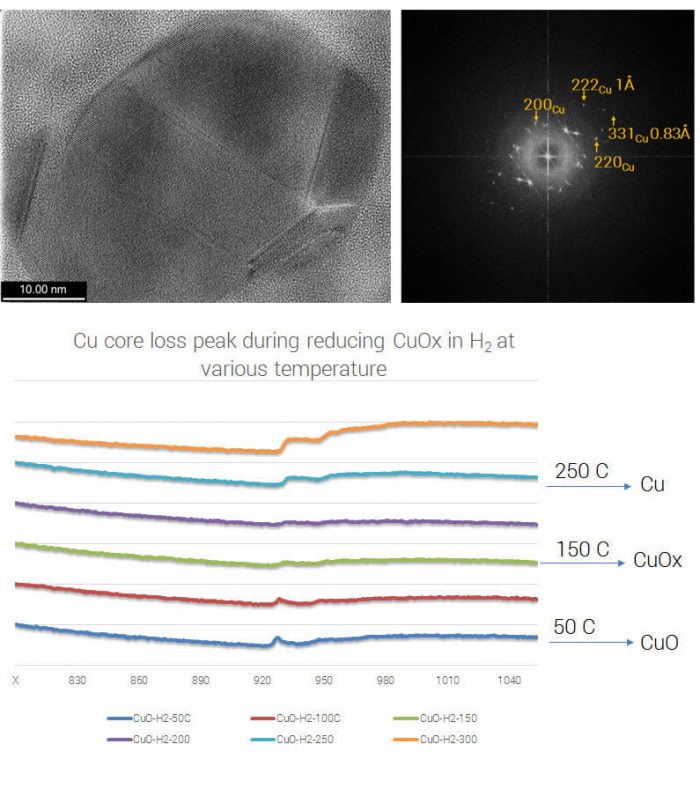
An understanding of the evolution of chemical bonding of catalysts under real world operating conditions can be generated using TEM-EELS down to the single particle level. Similar data can only otherwise be obtained using an in situ XPS at a synchrotron
Experiment: CuO exposed to 500 mBar H2:N2:O2 at 350°C HRTEM images (left) were taken at 300 °C with a resolution better than 1 Å (see FFT, right) after the sample was reduced from CuO in 1 bar pressure of H2 + 4N2. EELS measurements were taken at 50 °C increments, showing that the reduction of CuO to metallic Cu is achieved at 250 °C (lower) – data from Dr. Qiang Xu, DENSsolutions, The Netherlands.
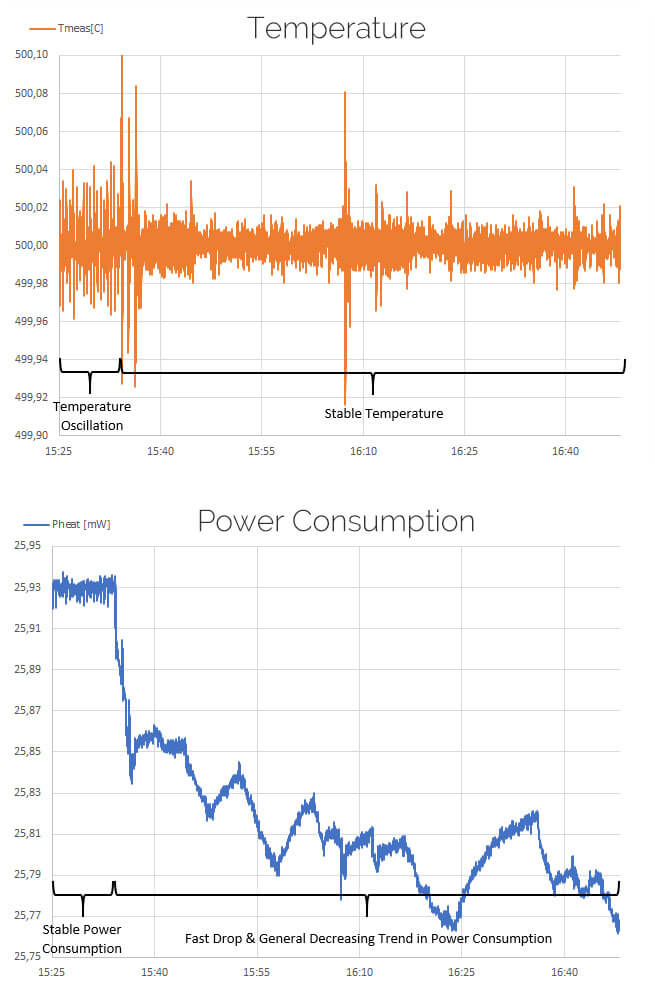
The high sensitivity 4-point-probe micro-heater used in the DENSsolutions Climate is ideal for measuring the energy emitted or absorbed as the result of an exothermic or endothermic reaction. Catalysts commonly have multiple reaction steps. Using the constant temperature mode of the Nano-Reactor, tiny changes in input energy can be measured, indivative of these reactions.
In this example in situ TEM observation of the CuO redox reaction and the phase transformation from Cu to Cu2O. At about 15:32min, energy consumption drops off, followed by period of oscillation with an overall downward trend. While the phenomenon is not fully understood, it is clear exothermic reactions are taking place.
Experiment: CuO exposed to 110 mBar MeOH at 500°C – data from Dr. Marc Willinger & Dr. Ramzi Farra, Fritz-Haber-Institute fur der Max-Planck-Gesellschaft, Germany.
