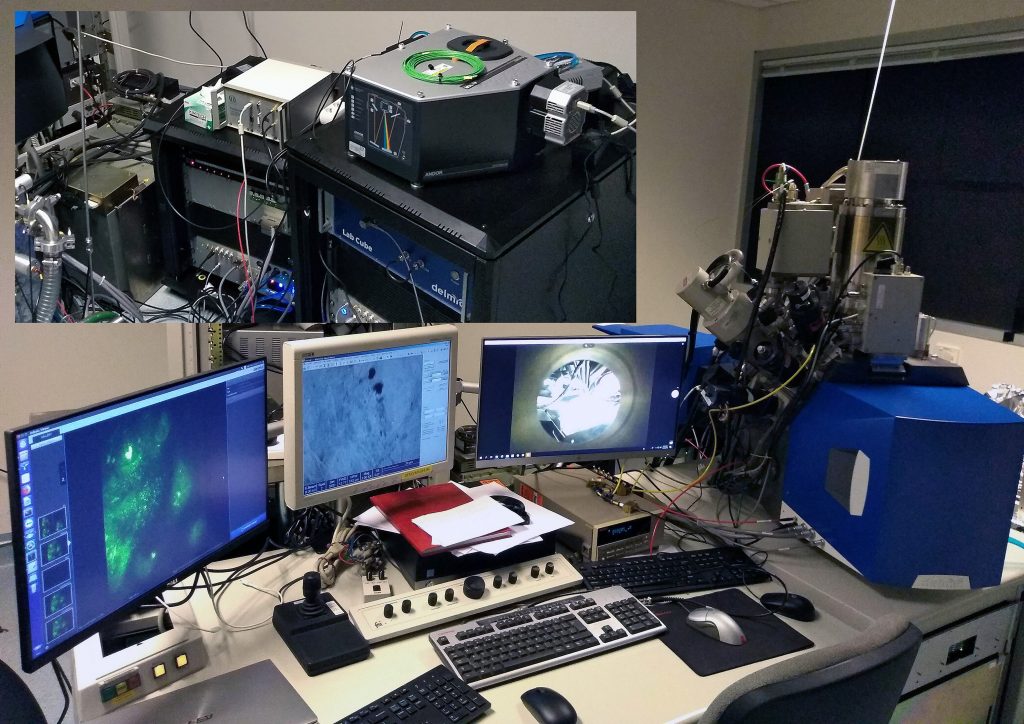
In this insightful interview we speak to Prof. Matthew Phillips from UTS about their recent Delmic installations. Their unque setup includes a DELMIC SPARC cathodoluminescence system installed on the same column as the DELMIC SECOM CLEM. He tells us about their rationale behind the the unique configuration, how they came to choose these systems and the groundbreaking research they will be doing.
You have a long history working with cathodoluminescence, even having built your own system. What is it about this technique that interests you so much?
The key strength of the CL technique is its capacity to non-destructively measure and image the optical and electrical properties of bulk and nano-structured materials and devices at the nano-scale in three-dimensions. Of particular importance, is the CL technique’s ability to study light emitting quantum structures, such as quantum wells, wires and dots. These quantum structures are typically sandwiched between other semiconductors, which rules out the use of photoluminescence measurements as the capping layer absorbs the laser excitation. CL measurements, however, are possible by simply increasing the SEM electron beam energy so that the injected electrons can traverse the top coating to excite light emission in the embedded quantum structures. This particular application is why we acquired out first CL system at UTS in 1991.

Additionally, many of the CL peaks in technologically important materials, can be attributed to the different chemical states of dopants and impurities as well as point defects, such as atomic vacancies. So the CL signal can be used to map their distribution and concentration at high magnification with high sensitivity, which can then correlated to spatial information provided by the SEM or other spatially resolved data physical or structural properties, from, for example, elemental X-ray microanalysis or EBSD results.
What was it about the DELMIC SPARC system that attracted you to it?
The DELMIC SPARC offered new CL techniques that had not been previously available in commercially available CL systems.
However, the main attraction was the SPARC’s outstanding light collection efficiency which is made possible by a high precision alignment system for the parabolic mirror that collects the CL signal. The SPARC also uses high performance low loss light transfer optics to the monochromators and detectors to maximise the measured CL signal.
The high CL collection efficiency means that the lower SEM accelerating voltages and electron beam currents can be used, which enables higher spatial resolution CL imaging and microanalysis.
However, the main attraction was the SPARC’s outstanding light collection efficiency which is made possible by a high precision alignment system for the parabolic mirror that collects the CL signal.
Prof. Matthew Phllips, UTS
The SPARC is capable of several different modes of CL. Which modes in particular are of interest to you?
The SPARC system was acquired to expand and enhance our existing CL Facilities at UTS. The new system has 3 high sensitivity CCD cameras that cover the UV, Visible and NIR spectral regions, measuring light with wavelengths ranging from around 180 to 1600 nm. And has 2 spectrometers, one that facilitates the new CL capabilities and the other high spectral resolution and NIR CL spectroscopy. I am also very interested in using and exploring the utility of new SPARC CL capabilities, in particular angle-resolved CL, time-resolved CL and polarization CL.

What experiments will you be able to do with the SPARC that you were not previously able to do and what sorts of materials will you be investigating?
The SPARC CL system will allow CL imaging and spectroscopy studies at high magnification with high CL collection efficiency over broader range of wavelengths from the UV to the NIR.
The new CL capabilities opens the door for a number of new experiments.
- Angle-resolved CL enables the measurement and mapping of the angle and intensity of the CL emission, which is important in nano-photonics as it enables studies of light-matter interactions at high magnification.
- Time-resolved CL, facilitates measurement and mapping on dopants, defects and impurities based on their CL emission lifetime instead of their emission energy or colour. The time-resolved CL also allow quantum spectroscopy measurements to detect and study single photon emitters and devices.
- Polarised CL uses to polarisation of the CL emission to image, measure and study light-matter interactions from nano-scale structures and materials.
How many other people are looking at using the SPARC system and what projects and materials are they looking to investigate?
The Delmic/SPARC system was funded by an ARC LIEF grant (LE180100030), with 13 chief investigators from 7 Universities, including UTS, RMIT, QUT, UNSW, USyd, Monash and Wollongong. The Delmic CL system will be used large number of projects involving the study of bulk and nano materials used in:
- Optoelectronics,
- Plasmonics,
- Nanophotonics,
- Quantum photonics,
- Nano-catalysts,
- Sensor development,
- Nano bio-photonics,
- Bio-imaging as well as
- Energy generation and energy storage technologies.
There was an application that the SPARC could theoretically perform. Since your system has been installed you have shown that it can be done. Can you tell us about it?
Photon bunching and anti-bunching spectroscopy measurements are typically conducted using sub-band gap laser excitation. Consequently, even though the SPARC’s time-resolved CL instrumentation was in principle capable of collecting these spectra, there was initially some debate over whether or not the anti-bunching measurement could be performed using electron beam excitation. So we were very pleased that during the Delmic compliance testing the CL correlation spectroscopy measurement was demonstrated on-site at UTS, using nanodiamond samples that exhibit single photon emission provided by Prof Igor Aharonovich.
You also installed a DELMIC SECOM CLEM system. What research was that system acquired for?
The Delmic SECOM CLEM platform integrates an optical fluorescence microscope into a SEM using a below sample objective lens with external light sources. Fluorescence imaging uses functionalised luminescence nanoparticles and dyes to selectively highlight specific regions in biological samples. The SECOM setup allows the microstructure of the labelled regions to be studied using the SEM at magnifications much higher than what is possible with conventional optical microscopy. We plan to use the SECOM for correlative SEM and fluorescence imaging studies of biological samples.
What features/capabilities of the SECOM system lead you down that part, given its unique configuration?
There were existing plans to build a below sample objective lens setup in a SEM to inject focussed laser light and collect photoluminescence in a SEM for correlative SEM, CL and PL measurements. We have already re-configured our SECOM to enable these measurements.
What projects are you aware of that your researchers will be using it for?
Besides the bio-research applications, the modified SECOM will be used to investigate the optoelectronic properties of nanophotonic and plasmonic nanostructures and emerging 2D materials, up-conversion nanocrystals and metamaterials.
You have installed the SECOM on the same FIB as the SPARC. Is this the only installation of its type in the world? And are there any experiments that you have in mind where the two techniques could be used correlatively?
I believe it is.
The correlative facility will enable a number of innovative experiments on:
- Multifunctional 2D materials for photonics and energy applications,
- Advanced metamaterials for optical systems, as well as
- Single photon emitters for Quantum Plasmonics and Nanophotonics applications.