CN Bio, a leading Organ-on-a-Chip (OOC) company that designs and manufactures single-and multi-organ microphysiological systems (MPS), today announced the commercial launch of the PhysioMimix™ Single-organ Higher Throughput (HT) System. The company’s first HT system has been designed to overcome adoption barriers currently limiting the use of predictive human liver models within drug discovery workflows to enable use within earlier stages where larger-scale comparative studies that investigate the efficacy, disposition or safety of lead candidate drugs are required.
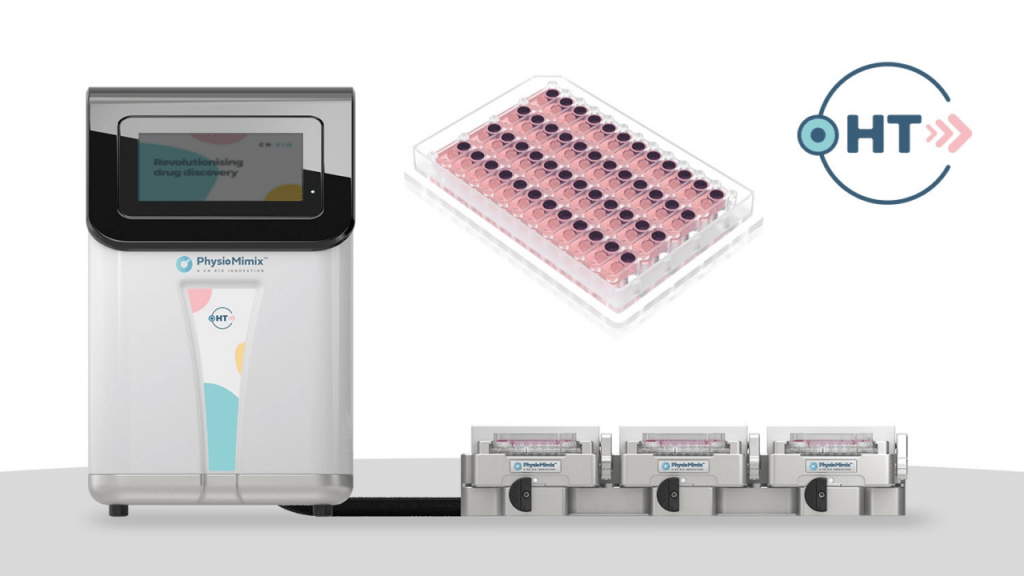
The PhysioMimix HT System provides users with a scalable solution, combining a significantly reduced cost-per-chip with increased run capacity. Launching alongside the system is a new multi-chip consumable plate, the Liver-48 plate, which faithfully miniaturizes CN Bio’s well-characterized and validated human liver model for applications that include predicting drug-induced liver injury or determining drug metabolism/hepatic clearance1 and the modelling of various prevalent liver diseases such as Non-alcoholic steatohepatitis (NASH)2.
Allowing 48 liver chip assays within one laboratory-standard SBS footprint, the plate supports the incorporation of replicates, controls and seven-point dose-response curves to improve data robustness and reproducibility. Multiple Liver-48 plates can be run simultaneously to provide a total capacity of 144 chips per HT System, with the potential to expand this further in response to consumer demand. Together, the new technology enables the benefits of human-relevant OOC insights to be realized earlier in drug discovery, facilitating more confident decision-making and the potential recovery of overlooked therapeutic candidates.
In support of New Approach Methodologies (NAMs) such as MPS, the FDA Modernization Act 2.0 has recently passed to remove the mandatory requirement for animal testing of developmental drugs for toxicity where enhanced performance is proven using in vitro alternatives3. The potential of MPS technology is further reinforced by the FDA’s decision to expand its collaborative research following several successful projects with CN Bio, most recently to investigate the company’s Gut/Liver model for human drug bioavailability studies4.
Dr Paul Brooks, CEO of CN Bio, said: “The timing of this launch is particularly pertinent following significant US legislative changes, which should alleviate another barrier to more widespread OOC adoption and emphasises the potential of MPS to revolutionise drug discovery processes. Representing the next generation of OOC technology, the PhysioMimix Single-organ HT System extends and complements our existing portfolio, enabling scientists to pick the solution, or solutions, that best meet their research needs and phase of drug discovery to bring more confident decision-making to the process and the potential recovery of flawed drugs.“
CN Bio’s PhysioMimix OOC range of MPS is designed to reliably bridge the gap between existing preclinical methods and human studies. Complementing the simplicity of traditional in vitro cell culture and the complexity of in vivo animal models, the company’s suite of hardware, consumables and assay protocols delivers accurate predictions where traditional approaches fall short. The MPS enables users to culture primary human cells as 3D microtissues in a fully perfused microenvironment that incorporates key elements of the human immune system. Faithfully recreating the physiology and function of human tissues and organs in an in vitro environment, they offer a path forward for the testing of new human-specific therapeutic modalities and the insights from MPS to be realized earlier and at more stages of drug discovery than achieved to-date.
- Characterizing the Reproducibility in using a Liver Microphysiological System-for Assaying Drug Toxicity, Metabolism and Accumulation.Rubiano et al., 2020
- Modelling human liver fibrosis in the context of non-alcoholic steatohepatitis using a microphysiological system Kostrzewski et al., 2021
- Congress Approves Landmark Measure to Reduce Animal (globenewswire.com)
- The FDA further expands collaboration with CN Bio to evaluate the PhysioMimix Multi-organ microphysiological system – CN Bio (cn-bio.com)
