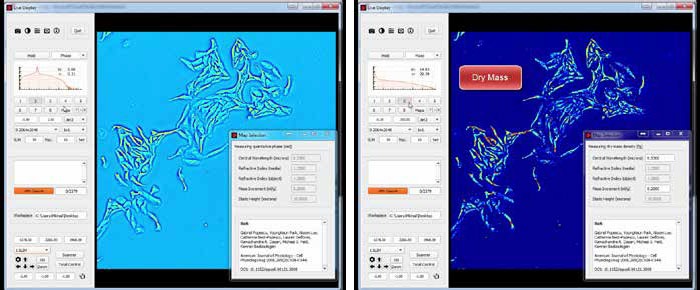
Phi Optics SLIM is a non-invasive phase imaging technology that quantifies the physical properties of live cells and tissues. The output is a live quantitative image (SLIM map) of the specimen. The intensity of every pixel in the frame is a measure of a phase shift map (in radians) or the optical path length difference (in nanometers) through the sample, which is measured with sensitivity better than 0.5 nanometers. As shown in Figure 1, the phase shift map is converted on-the-fly to other SLIM maps, with their respective pixel intensity: thickness (in microns), dry mass area density (picograms per square micron) and refractive index. Moreover, SLIM is capable of automatically scanning a blood smear slide at high resolution, allowing for an automated blood testing. For researchers with existing inverted light microscopes, SLIM can be easily added to most systems regardless of manufacturer.
SLIM Features
- Live and timelapse imaging
- Programmable 2D and 3D scanning using the microscope full range of motion
- Seamless multi-channel registration
SLIM Imaging
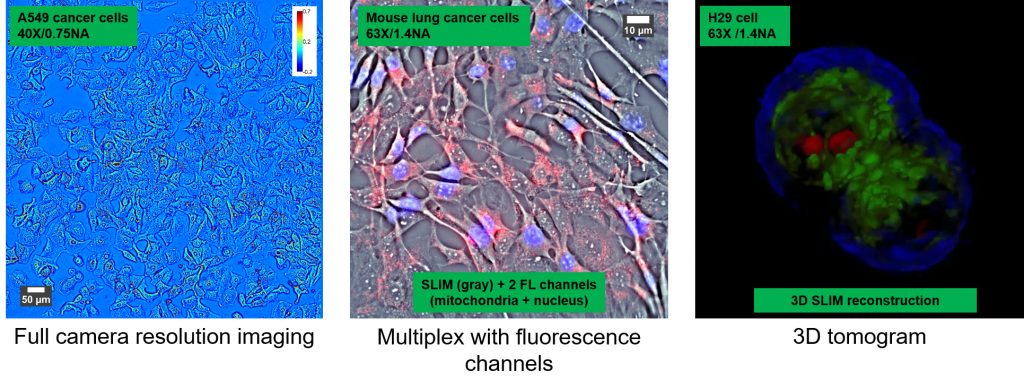
The SLIM approach to quantitative phase imaging provides speckle-free images due to high sensitivity of the measurement (nanometer scale spatial noise). Submicron optical sectioning is facilitated by high NA objectives and the micron-scale coherence length of the illumination – SLIM can render 3D tomographic images of transparent structures just by scanning the specimen through focus (Z-scanning). The design modularity enables multiplexing with fluorescence imaging for multimodal, in-depth biological studies.
Imaging live cells using classical brightfield microscopy is notoriously difficult because they absorb and scatter very little light. Fluorescence microscopy employs fluorophores which absorb and emit light, rendering the cells visible. The fluorophores can be genetically encoded or injected into the live cells and their location and emission intensity is used to accurately quantify the cell features and processes. Continuous imaging is possible only for short periods of time (time-lapse) to avoid phototoxicity, photo bleaching and measurement bias.
Live unstained cells exhibit gradients of optical path length (i.e. product of thickness and refractive index) across their structure. Phase contrast (PC) and differential interference microscopy (DIC) modalities convert minute changes in optical path length into differences in brightness (amplitude contrast). These techniques are not intrinsically quantitative or specific and lack the resolution of fluorescence microscopy but continuous long-term imaging is possible because of the low levels of illumination required.
The SLIM Operating Principle
Phi Optics SLIM is a non-invasive phase imaging technology that quantifies the optical path length differences in a biospecimen and converts them into thickness, dry mass area density and refractive index maps. Figure 2 illustrates the principle of technology in its reference implementation. A live cell in culture medium is imaged with the phase contrast modality of the microscope.
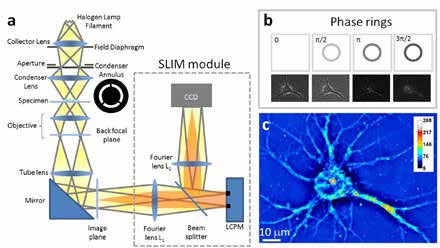
The light passing through the object (scattered beam) and the light passing through the medium (reference beam) combine through interference in the image plane. The optical path length differences between the beams (i.e. the phase shift) in each point of the image plane are converted into brightness differences. Optically dense areas of the cell (e.g. nucleus) introduce a phase shift of up to – 0.5π radians in the scattered beam with respect to the reference beam. In the most common phase contrast implementation, before reaching the image plane the reference beam also undergoes a + 0.5π radians phase shift by passing through a phase plate: a glass ring with finite thickness in the back focal plane of the objective. Destructive interference generates a dark image for the dense portions of the cell with respect to the grey background (see Figure 3b).
The SLIM module relays the image plane with minimal aberrations (diffraction limited) at a 1:1 ratio to a camera sitting at its exit port. The active element at the heart of the SLIM module is a liquid crystal spatial light modulator (SLM). The SLM is conjugated with the back focal plane of the microscope objective, and it modulates the reference beam like a phase plate with variable thickness. To create a quantitative phase image the SLM shifts the phase of the reference beam by a fixed amount (0, 0.5π, π, 1.5π) and the camera captures the resulting frame (Figure 3b). The CellVista software module combines the four frames by solving the field interference equations in each point of the frame – the result (Figure 3c) is a quantitative-phase (SLIM) image that is uniquely determined.
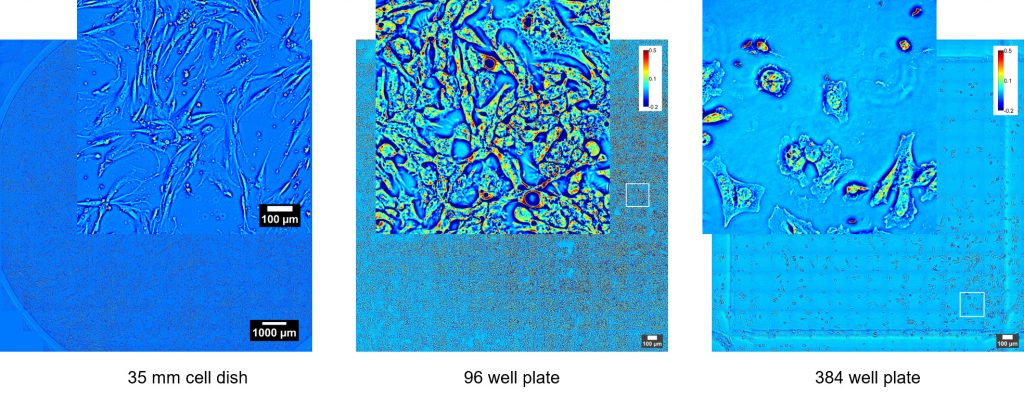
SLIM is a wide field quantitative imaging method thus it can measure simultaneously large populations of cells at full camera resolution (e.g. 2 mm FOV for 10X objective at 4.2 MP camera resolution). Wide field optical sectioning (e.g. 850 nm Z-resolution for 100X/1.4NA objective) enables 3D tomography. All microscope output is acquired with the same camera which enables seamless overlay of SLIM images with fluorescence channels.
SLIM Configuration
Phi Optics patented SLIM technology employs optical interferometry for extreme sensitivity to structure and dynamics. Phi Optics implements SLIM as an add-on to all major brand optical microscopes (10X to 100X magnifications) (see Figure 3).
Quantitative Imaging Capabilities Afforded by SLIM
By upgrading your existing microscope with the CellVista-SLIM, you will add the following 4D label-free quantitative imaging capabilities:
- Quantitative cell assays: growth and proliferation, cytotoxicity, viability, immune cell killing, senescence
- Digital pathology slides: tissue morphology and refractive index mapping with or without staining
- Size, count and 3D morphology of protein particles
SLIM Examples