Intravital imaging is essential for cellular-level studies in live animal research, but capturing high-resolution images of deep tissues is challenging due to organ pulsation and animal movement, which can displace imaging areas. In this article we share insights from IVIM Technology customers conducting real-time in vivo experiments on live mice. It highlights the difficulties encountered when imaging highly dynamic organs such as the heart, lungs, thymus and uterus. It also explains how IVIM Technology’s Tissue Motion Stabiliser (TMS) and advanced imaging chamber systems, along with user-friendly imaging techniques, effectively address these challenges.
Minimising Subtle Micron-Level Movements
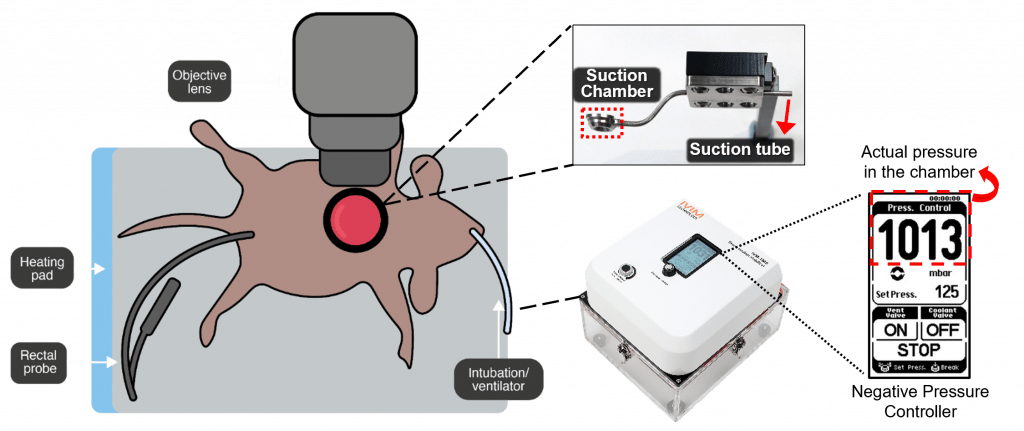
The primary challenge in imaging dynamic organs is minimising subtle micron-level movements that can obstruct clear observation. As a pioneering inovative solution, to overcome the difficulties associated with tissue motion, IVIM Technology have developed a Tissue Motion Stabiliser (TMS) which uses negative pressure to securely hold pumping organs in place, ensuring stable imaging conditions and minimising motion artifacts. By applying suction to the tissue, the TMS system restrains motion, allowing for high-quality imaging even in dynamic environments. It features a circular metal fixture and a suction pump that work together to stabilise the observation site and mitigate the impact of pulsations (Fig. 1).
Applications of TMS Utilisation for Dynamic Organ Imaging In Vivo

The TMS system is essential in intravital imaging, addressing the challenges of tissue motion in highly dynamic organs such as the heart, lungs, thymus, and uterus. The heart, which is deeper within the body, requires a specialised approach with stronger negative pressure to effectively visualise it, unlike the more flexible lung tissue. Similarly, the rhythmic contractions of the uterus and thymus complicate the capture of clear intravital images using conventional methods. To address these challenges, our vacuum-suction chamber for imaging the uterus, thymus, lung and heart incorporates several innovative modifications designed to overcome these obstacles.
In Vivo Lung Imaging of Live Mouse Utilising TMS
Lung Imaging Chamber with IVM-TMS marks a significant advancement in preclinical research, particularly for studying live mouse. The imaging chamber features fine adjustment controls for precise tissue sample positioning, optimising conditions for accurate imaging. This setup enables detailed observation of blood flow and immune cell mobility in live lung imaging.
Connectivity between the chamber and the IVM-TMS system, via tubing, allows for adjustable airflow and negative pressure, effectively stabilising the tissues.
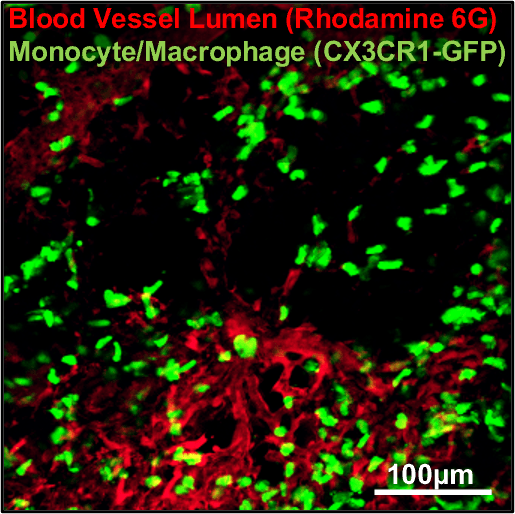
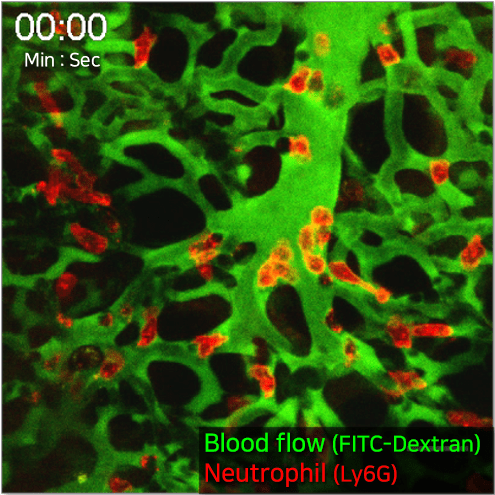
By employing IVM-TMS in conjunction with intravital microscopy, users can detect distortions in pulmonary microcirculation resulting from sepsis-induced acute lung injury (ALI). This technique allows for the exploration of underlying cellular pathophysiological mechanisms and enables visualisation of both pulmonary microcirculation and circulating cells in vivo within the anesthetised live mouse lung.
In Vivo Heart Imaging of Live Mouse Utilising TMS
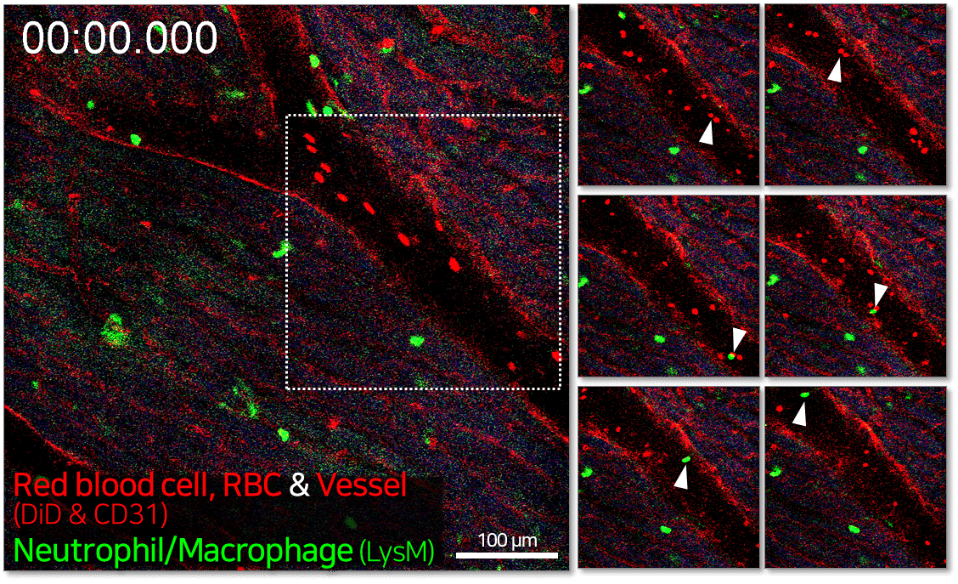
Unlike the relatively soft and deformable lung tissue, the heart, being deeper in the body, requires stronger negative pressure for effective visualisation. In this example, LysM-eGFP transgenic mice were used to visualise immune cell dynamics, with vascular labeling achieved through intravenous injection of anti-CD31 antibody and DiD-labeled red blood cells (RBCs). After a chest incision exposed the cardiac tissue, an imaging window chamber with a vacuum-based tissue motion stabiliser set to 890–920 mbar was used to stabilise the tissue and enable high-quality heart imaging.
Ref: European Heart Journal – Imaging Methods and Practice (2024) 2, qyae062
In Vivo Thymus Imaging of Live Mouse Utilising TMS
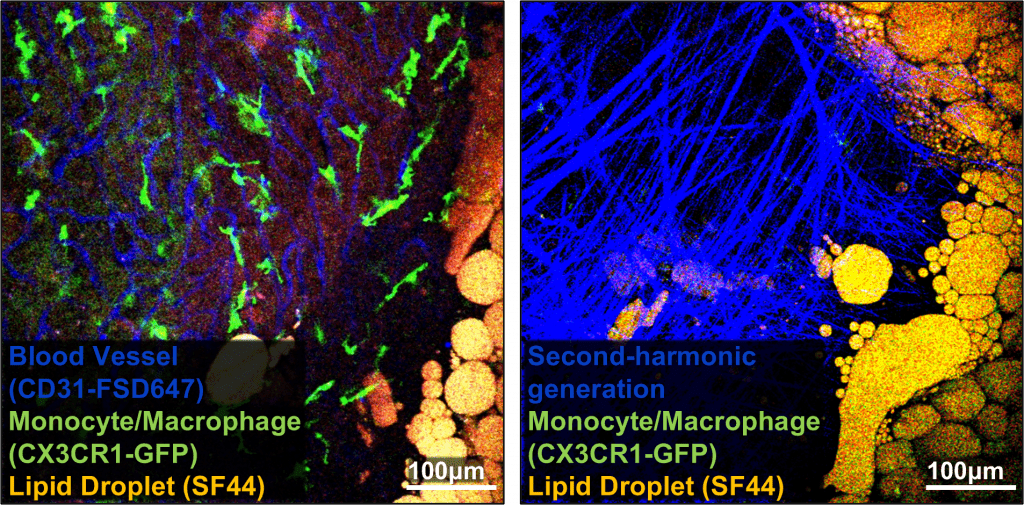
Challenges arise from the delicate nature of thymic tissue and its proximity to the heart. To address these issues and capture detailed images, TMS was employed, which provides the necessary negative pressure to stabilise the tissue during imaging. This study focuses on dynamically monitoring immune cell behavior within its microenvironment using IVM-TMS. It has shed light on the complex interactions between dietary factors, immune cell behavior, and thymic vasculature dynamics. By combining Second-Harmonic Generation (SHG) imaging with GFP expression in immune cells, we gained a comprehensive understanding of the interplay between immune cell behavior and thymic structure.
In Vivo Uterus Imaging of Live Mouse Utilising TMS

The natural rhythmic contractions of the uterus have historically posed significant challenges for capturing high-quality intravital images, often resulting in blurry or unclear images with traditional methods. Our Uterus Imaging System addresses these issues with a specially designed vacuum-suction chamber (Fig. 1) that incorporates several innovative modifications to stabilize the uterus during imaging. This advanced chamber effectively mitigates the effects of uterine contractions, enabling researchers to achieve clear, high-resolution and consistent imaging at the same location.
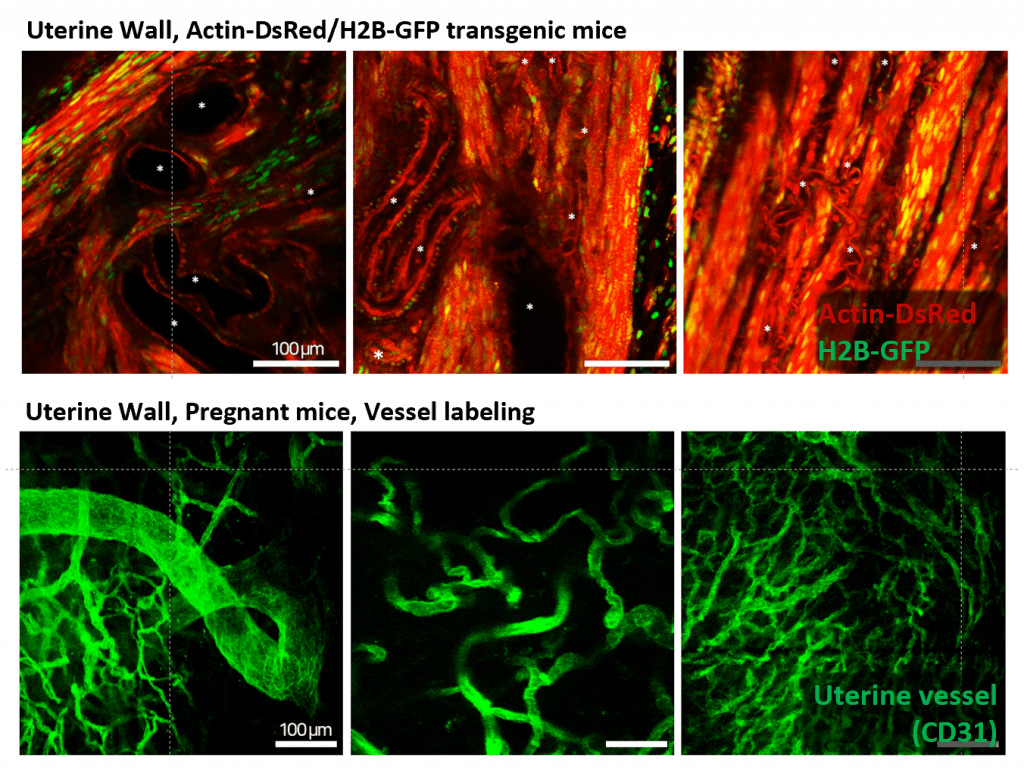
The uterus is composed of muscular tissue, enabling it to sustain pregnancy and facilitate childbirth. Throughout these processes, it is crucial for the uterus to receive an adequate blood supply. By observing the muscular tissue and blood vessels (Fig. 7) in the uterus of mice before and during pregnancy, we obtained results that enhance our understanding of the structural changes in the uterus due to pregnancy and the diseases that may occur during pregnancy.
Conclusion
In conclusion, the introduction of vacuum-suction chambers with IVM-TMS marks a significant advancement in intravital imaging technology. By overcoming inherent challenges in visualizing dynamic organs in vivo, this innovation opens new frontiers in physiological research and biomedical imaging, promising groundbreaking discoveries.
